Avigan “clearly effective” in treating COVID-19 says Chinese official
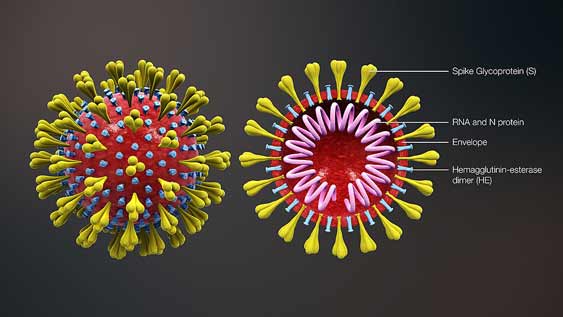
Due to the increasing outbreak of Corona virus worldwide, scientists all over the world are engaged in developing its medicine. The US has reached the testing stage after developing the drug. Meanwhile, a Chinese official has claimed that the Avigan anti-flu drug of the Japanese company Fujifilms is proving effective on patients with coronavirus. Following the Chinese official’s statement on Wednesday, the company’s stock closed up 15.4 percent at 5238 yen. Avigan is also known as Favipiravir.
Japan approved the use of this medicine in 2014. Zhang Jinmin, an official of China’s Ministry of Science and Technology, says that this drug has not only proved to be effective on coronavirus, but also no side effects have been seen so far. According to the information, this drug was tested on 340 patients in Shenzhen and Wuhan. After some time, the test of corona virus of these patients came negative. All of them were started been given this medicine four days after their coronavirus test was positive.
After giving this medicine, the X-Ray report of these patients also saw improvement in their lungs up to 91 percent. At the same time, in those who were given a second medicine instead, 61 percent of the patients’ lungs were found better than before. The simplest meaning to say is that this drug has proved to be more effective than other medicines on the coronavirus patients. However, Japan has so far not made any claims on its part to succeed in this way.
The Guardian newspaper quoted Japanese health ministry sources as saying that the drug was also given to patients suffering from the coronavirus in Japan, but it did not work well on patients there. According to this, about 70-80 patients were given this medicine, but it did not appear to have any benefit on the patients. According to this report, in 2016, the Japanese government had supplied Avigan as an emergency medicine in Guinea after the Ebola virus reached many countries of the world. Favipiravir had sought permission from the government to use it on full scale on Covid-19 patients.